Page 20 - Kimya 11 | Çalışma Defteri
P. 20
Beceri Temelli - II
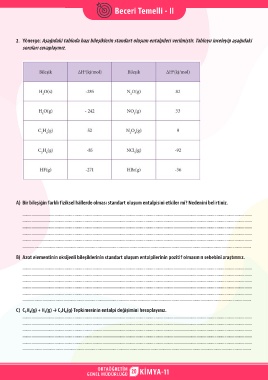
2. Yönerge: Aşağıdaki tabloda bazı bileşiklerin standart oluşum entalpileri verilmiştir. Tabloyu inceleyip aşağıdaki
soruları cevaplayınız.
A) Bir bileşiğin farklı fiziksel hâllerde olması standart oluşum entalpisini etkiler mi? Nedenini belirtiniz.
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
B) Azot elementinin oksijenli bileşiklerinin standart oluşum entalpilerinin pozitif olmasının sebebini araştırınız.
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
C) C₂H₄(g) + H₂(g) → C₂H₆(g) Tepkimesinin entalpi değişimini hesaplayınız.
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
...............................................................................................................................................................................................................
ORTAÖĞRETİM 20 KİMYA-11
GENEL MÜDÜRLÜĞÜ